Check your pipes: the sewage system of the brain
Your brain is full of trash—and I don’t mean the details of every episode of the new season of Love is Blind or the latest trend on TikTok. More like the debris from dead cells and broken-down proteins. How do our bodies clear it out so that our brains don’t end up looking like New York City sidewalks? Turns out, a sophisticated network of channels helps to extract and drain waste from brain tissue, almost like pipes in a sewer system.
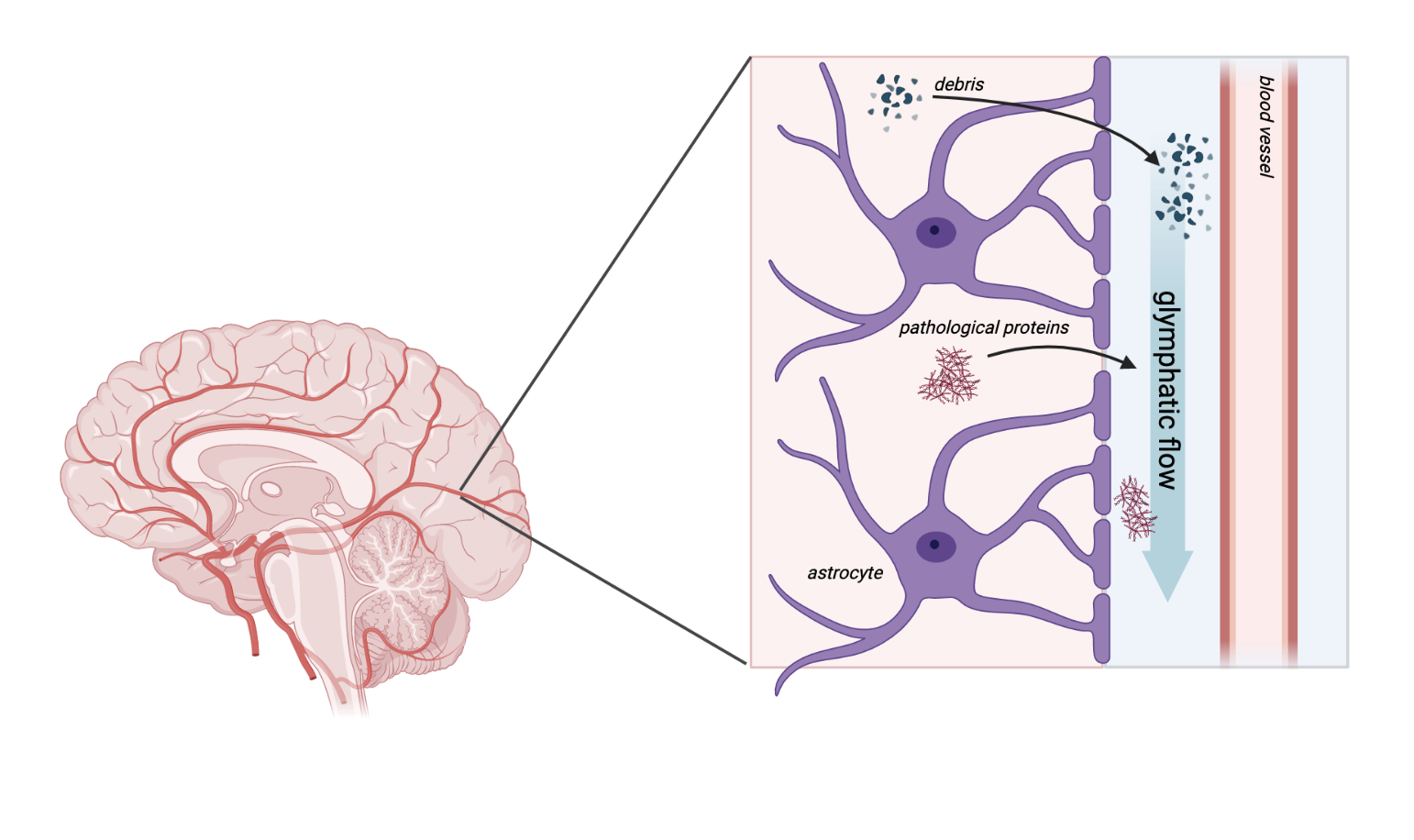
This network is called the glymphatic system, a combination of the word “glia,” which are the support cells of the brain, and “lymphatic,” a system that similarly coordinates waste disposal and immune response in the rest of the body. A type of glia called astrocytes wrap protrusions called endfeet around blood vessels in the brain, creating a space around the vessel through which cerebrospinal fluid (CSF) flows. CSF helps to deliver nutrients to brain tissue as well as draining waste.
Researchers can study glymphatic function by using fluorescent or radioactive molecules, which they can trace as they move through and exit the brain. Alternatively, they can measure the amount of proteins in the brain as they are flushed out over time. They can also use specialized brain imaging techniques to visualize fluid exchange in different brain regions.
Sleep appears to be an important component of healthy glymphatic function. Researchers have shown that the channels through which waste is drained widen by 60% during sleep, increasing fluid volume and the clearance of waste, including a protein called amyloid beta that is involved in Alzheimer’s disease pathology[1]. Interestingly, evidence suggests that dysfunctional sleep may be a risk factor for Alzheimer’s disease and could worsen its progression[1,2].
Pathological protein buildup is characteristic of several diseases and disorders, in fact, including Alzheimer’s disease, other dementias, Parkinson’s disease, Huntington disease and amyotrophic lateral sclerosis (Lou Gehrig’s disease). It has been proposed that reduced clearance of these proteins with impaired glymphatic function could contribute to or worsen disease progression in all of these[3,4,5,6,7,8], and this is a driving motivation for improving our understanding of the glymphatic system. While there may not be teenage mutant ninja turtles living in the sewers of our brains, the glymphatic system is still an exciting area of study and could have important implications for our understanding of disease.
References
Xie, L. et al. (2013) Sleep Drives Metabolite Clearance from the Adult Brain. Science 342, 373–377
Shokri-Kojori, E. et al. (2018) β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. 115, 4483–4488
Nedergaard, M. and Goldman, S.A. (2020) Glymphatic failure as a final common pathway to dementia. Science 370, 50–56
Majumder, V. et al. (2018) TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis. BMC Neurol. 18, 90
Ding, X.-B. et al. (2021) Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 27, 411–418
Donahue, E.K. et al. (2021) Global and Regional Changes in Perivascular Space in Idiopathic and Familial Parkinson’s Disease. Mov. Disord. 36, 1126–1136
Caron, N.S. et al. (2021) Mutant Huntingtin Is Cleared from the Brain via Active Mechanisms in Huntington Disease. J. Neurosci. 41, 780–796
Chan, S.T. et al. (2020) Association of dilated perivascular spaces and disease severity in patients with Huntington’s disease. Neurology DOI: 10.1212/WNL.0000000000011121